Chemistry, 06.05.2020 07:02 kaliloabousjbf
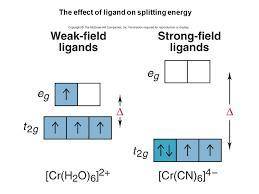
For the following give (1) oxidation # of metal, (2) number of d electrons, draw valence bond description of the complex, fill in metal and ligand valence electrons, give (3) metal orbitals that are hybridized, (4) type of hybridization, (5) molecular geometry, and (6) draw the crystal field de- scription of the octahedral complexes for a), c), and e).
[Cr(H2O)6]2+ (High spin)
(1) ___ (2) (3) (4) (5)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1


Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

You know the right answer?
For the following give (1) oxidation # of metal, (2) number of d electrons, draw valence bond descri...
Questions

Mathematics, 06.04.2020 01:38

Mathematics, 06.04.2020 01:38


Mathematics, 06.04.2020 01:38

Mathematics, 06.04.2020 01:39


Social Studies, 06.04.2020 01:39


Geography, 06.04.2020 01:39






Geography, 06.04.2020 01:40



Mathematics, 06.04.2020 01:40

History, 06.04.2020 01:40