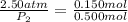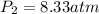Chemistry, 06.05.2020 07:10 tannercarr7428
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon dioxide) into the inflexible can. If a soda bottle has an initial pressure of 2.50 atm and 0.150mol of CO2, then is shaken up so there are now 0.500 moles of carbon dioxide, what will the new pressure of the soda bottle be?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

You know the right answer?
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon d...
Questions






Mathematics, 12.11.2019 22:31


Biology, 12.11.2019 22:31


English, 12.11.2019 22:31










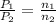
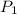 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm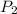 = final pressure of gas = ?
= final pressure of gas = ?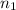 = initial number of moles of gas = 0.150 moles
= initial number of moles of gas = 0.150 moles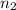 = final number of moles of gas = 0.500 mol
= final number of moles of gas = 0.500 mol