A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g...

Chemistry, 06.05.2020 07:23 davelopez979
A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g). ΔH = 560 kJ
1) This reactions is .
O Endothermic
O Exothermic
2) Suppose 94.2g of Fe2O3 react. will any heat be relased or absorbed?
O Yes, absorbed.
O Yes, released.
O No.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1
You know the right answer?
Questions

Computers and Technology, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00


Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Health, 02.12.2020 23:00



Health, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00




Biology, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

History, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00



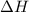 for the reaction comes out to be positive.
for the reaction comes out to be positive.

