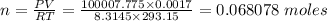Chemistry, 06.05.2020 07:40 persondontknowhelp
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid whose vapor pressure is the same as pure water at 20.0 degrees C (18 torr). The volume of the mixture is 1.7 L and its total pressure is 0.810 atm. Determine the number of moles of hydrogen gas present in the sample.
A. 0.272 mol
B. 0.04 mol
C. 0.997 mol
D. 0.139 mol
E. 0.0681 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid who...
Questions

Chemistry, 06.10.2020 14:01

Biology, 06.10.2020 14:01


Social Studies, 06.10.2020 14:01

History, 06.10.2020 14:01












Mathematics, 06.10.2020 14:01

History, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Biology, 06.10.2020 14:01