
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)3 with 1.01.0 mL of 0.00200.0020 M KSCN. KSCN. The equation for the reaction is as follows. Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO−3 Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO3− What allows us to assume that the reaction goes essentially to completion? The reaction quotient Q is greater than Kc. Kc. The concentration of Fe(NO3)3Fe(NO3)3 is much higher than the concentration of KSCN. KSCN. The excess Fe3+Fe3+ prevents the formation of the neutral Fe(SCN)3.Fe(SCN)3. The equlibrium reaction has a very high Kc. Kc. Under the conditions given, Le Châtelier's principle dictates that the reaction shifts to the left. Based on that assumption, what is the equilibrium concentration of FeSCN2+?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1

Chemistry, 23.06.2019 13:30
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1
You know the right answer?
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)...
Questions

Advanced Placement (AP), 12.04.2021 21:00



History, 12.04.2021 21:00








Chemistry, 12.04.2021 21:00








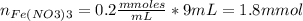
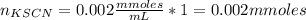
![[Fe(NO3)3]=\frac{1.8}{10} =0.18M](/tpl/images/0646/9896/2543f.png)
![[KSCN]=\frac{0.002}{10} =0.0002M](/tpl/images/0646/9896/5abd0.png)



