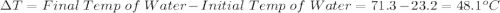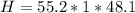Chemistry, 06.05.2020 06:05 fdougie111
You are running a calorimetry experiment where you are trying to determine the number of Calories (with a capital C!) in a peanut. You set up your aluminum can of water and take all your initial data, putting it in the table below. Then, you set your peanut ON FIRE You finish filling out your table once the peanut has gone out. How many Calories of heat did your peanut release? Round your answer to two digits after the decimal point.
Initial Mass of Peanut 3.11 grams
Final Mass of Peanut 0.52 grams
Mass of Water 55.2 grams
Initial Temp of Water 23.2 degrees C
Final Temp of Water 71.3 degrees C

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
You are running a calorimetry experiment where you are trying to determine the number of Calories (w...
Questions

Chemistry, 13.04.2022 07:40


Mathematics, 13.04.2022 07:40




Mathematics, 13.04.2022 07:50



Biology, 13.04.2022 07:50

English, 13.04.2022 07:50

Chemistry, 13.04.2022 08:00

Mathematics, 13.04.2022 08:00

Physics, 13.04.2022 08:00






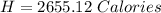
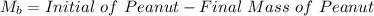
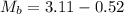
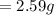
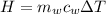
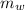 is the mass of water which is given as
is the mass of water which is given as 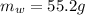
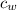 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 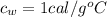
 is the change in temperature which can be evaluated as follows
is the change in temperature which can be evaluated as follows