

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Glycine has pKa values of 2.34 and 9.60. Therefore, glycine will exist predominately as a zwitterion...
Questions

Mathematics, 28.01.2021 18:00


Physics, 28.01.2021 18:00

History, 28.01.2021 18:00

Mathematics, 28.01.2021 18:00

Social Studies, 28.01.2021 18:00

Mathematics, 28.01.2021 18:00

Chemistry, 28.01.2021 18:00

Mathematics, 28.01.2021 18:00

World Languages, 28.01.2021 18:00

Computers and Technology, 28.01.2021 18:00

Business, 28.01.2021 18:00



English, 28.01.2021 18:00

English, 28.01.2021 18:00

Mathematics, 28.01.2021 18:00

Mathematics, 28.01.2021 18:00


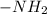 and -COOH should bear equal and opposite charges in it's zwitterionic structure.
and -COOH should bear equal and opposite charges in it's zwitterionic structure.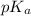 of -COOH (in glycine) = 2.34
of -COOH (in glycine) = 2.34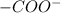 .
.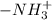 .
.

