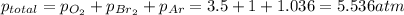Chemistry, 06.05.2020 06:32 nbunny7208
A metal tank contains three gases: oxygen, argon, and bromine. If the partial pressures of the three gases in the tank are 3.5 atm of O2, 760.0 mmHg of
Br2,and 105 kPa of Ar, what is the total pressure inside of the tank in atmospheres?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
A metal tank contains three gases: oxygen, argon, and bromine. If the partial pressures of the three...
Questions

Mathematics, 02.03.2021 18:40

Mathematics, 02.03.2021 18:40





Mathematics, 02.03.2021 18:40



Mathematics, 02.03.2021 18:40



Mathematics, 02.03.2021 18:40

Mathematics, 02.03.2021 18:40


Mathematics, 02.03.2021 18:40

Mathematics, 02.03.2021 18:40

Law, 02.03.2021 18:40

History, 02.03.2021 18:40

Social Studies, 02.03.2021 18:40