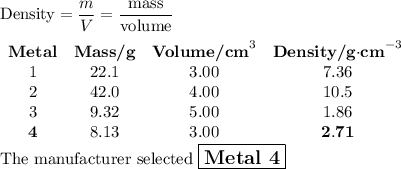
A manufacturer selected a metal to use in producing a lightweight button for clothing. A metal
...

Chemistry, 06.05.2020 05:08 ethanyayger
A manufacturer selected a metal to use in producing a lightweight button for clothing. A metal
that has a density of 2.71 g/cm was selected.
Metal Data
Metal
Mass (9)
22.1
42.0
9.32
8.13
Volume (cm)
3.00
4.00
5.00
3.00
3
Which of the metals was selected?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Questions

Computers and Technology, 10.03.2020 07:04







Chemistry, 10.03.2020 07:04

History, 10.03.2020 07:04

English, 10.03.2020 07:04


English, 10.03.2020 07:05



Mathematics, 10.03.2020 07:05

English, 10.03.2020 07:05

Mathematics, 10.03.2020 07:05

Mathematics, 10.03.2020 07:05