Chemistry, 06.05.2020 05:27 leslie1811
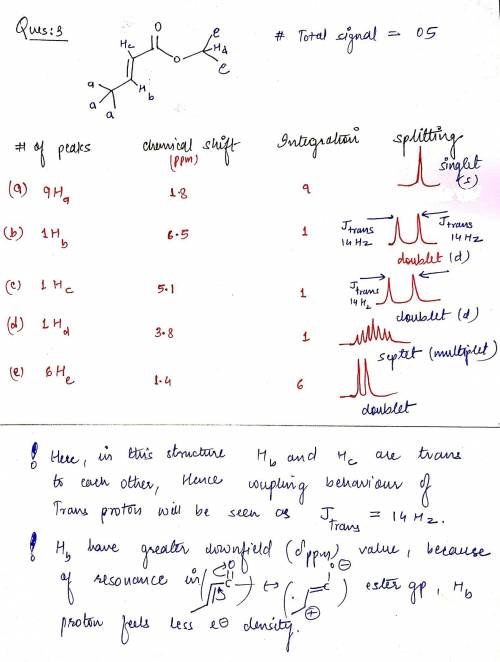
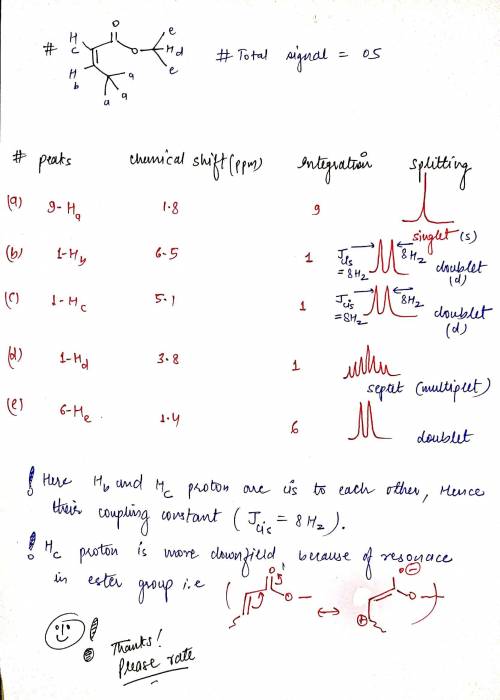
3. Consider the following stereoisomers of isopropyl methacrylate. Provide the expected number of peaks, the integrations of those peaks, the chemical shifts and the spliiting patterns expected for each. How are they different? b) Next to your description of the expected splitting pattern, please draw what that peak would look like. c) How could 1H NMR be used to determine the difference between these isomers? Please be specific.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Why is it illegal to manufacture fireworks without a license
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
3. Consider the following stereoisomers of isopropyl methacrylate. Provide the expected number of pe...
Questions



Mathematics, 24.07.2020 04:01

Mathematics, 24.07.2020 04:01



Mathematics, 24.07.2020 04:01


Mathematics, 24.07.2020 04:01

History, 24.07.2020 04:01




Biology, 24.07.2020 04:01





Mathematics, 24.07.2020 04:01

Chemistry, 24.07.2020 04:01