Chemistry, 06.05.2020 04:11 addisonrausch
One reaction involved in the conversion of iron ore to the metal is FeO(s) + CO(g) → Fe(s) + CO2(g) Use Hess’s Law to calculate ΔHrxn given the following. 3 Fe2O3(s) + CO(g) → 2 Fe3O4(s) + CO2(g) ΔH = −47.0 k Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) ΔH = −25.0 k Fe3O4(s) + CO(g) → 3 FeO(s) + CO2(g) ΔH = 19.0 k

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3



Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
One reaction involved in the conversion of iron ore to the metal is FeO(s) + CO(g) → Fe(s) + CO2(g)...
Questions



History, 14.01.2021 04:10

Mathematics, 14.01.2021 04:10






History, 14.01.2021 04:10


English, 14.01.2021 04:10

Biology, 14.01.2021 04:10

Mathematics, 14.01.2021 04:10





English, 14.01.2021 04:10

Mathematics, 14.01.2021 04:10

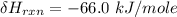
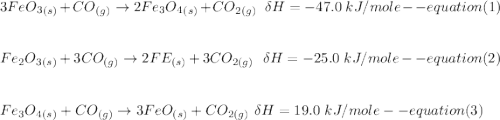
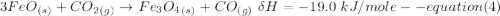
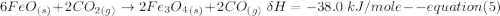
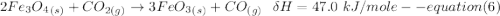
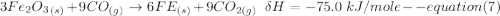

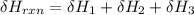 (According to Hess Law)
(According to Hess Law)