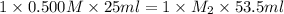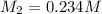Chemistry, 05.02.2020 08:02 sascsl2743
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 53.5 ml. what is the value of m2 for the reaction?
0.439 m
0.500 m
0.234 m
1.07 m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final sol...
Questions

SAT, 08.12.2021 17:10

Biology, 08.12.2021 17:10

English, 08.12.2021 17:10

Mathematics, 08.12.2021 17:10

History, 08.12.2021 17:10

History, 08.12.2021 17:10



History, 08.12.2021 17:10


Mathematics, 08.12.2021 17:10

Mathematics, 08.12.2021 17:10


Mathematics, 08.12.2021 17:10

English, 08.12.2021 17:10

Mathematics, 08.12.2021 17:10


Mathematics, 08.12.2021 17:10


World Languages, 08.12.2021 17:10

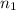 = basicity of an acid = 1
= basicity of an acid = 1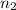 = acidity of a base = 1
= acidity of a base = 1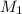 = concentration of KHP = 0.500 M
= concentration of KHP = 0.500 M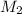 = concentration of NaOH = ?
= concentration of NaOH = ?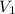 = volume of KHP = 25 ml
= volume of KHP = 25 ml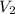 = volume of NaOH = 53.5 ml
= volume of NaOH = 53.5 ml