
Chemistry, 06.05.2020 04:28 jaylanmahone223
Combustion of hydrocarbons such as hexane (C6H14) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid hexane into gaseous carbon dioxide and gaseous water. x1 2. Suppose 0.290 kg of hexane are burned in air at a pressure of exactly 1 atm and a temperature of 13.0 °C. Calculate the volume of carbon dioxide gas that is produced Round your answer to 3 significant digits OL

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Combustion of hydrocarbons such as hexane (C6H14) produces carbon dioxide, a "greenhouse gas." Green...
Questions




English, 08.04.2020 20:25


Law, 08.04.2020 20:26


Mathematics, 08.04.2020 20:26




Chemistry, 08.04.2020 20:26


Physics, 08.04.2020 20:26

English, 08.04.2020 20:26


History, 08.04.2020 20:26

Social Studies, 08.04.2020 20:26


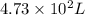
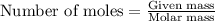
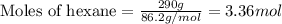
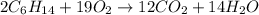
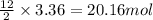 of carbon dioxide
of carbon dioxide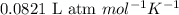
![13^oC=[13+273]K=286K](/tpl/images/0646/2755/9bc5c.png)



