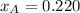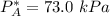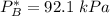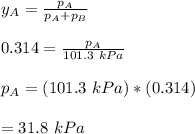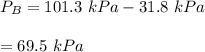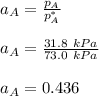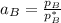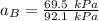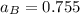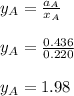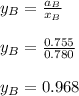Chemistry, 06.05.2020 03:07 rachel2005smith
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, it was found that xA=0.220 when yA=0.314. Calculate the activities and activity coefficients of both components in the solution. The vapor pressures of the pure components at this temperature are 73kPa and 92.1kPa.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, i...
Questions





Biology, 01.10.2019 22:40


History, 01.10.2019 22:40


Mathematics, 01.10.2019 22:40




Arts, 01.10.2019 22:40


Mathematics, 01.10.2019 22:40

Mathematics, 01.10.2019 22:40

Biology, 01.10.2019 22:40



Mathematics, 01.10.2019 22:40

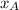 is the mole fraction in the liquid
is the mole fraction in the liquid 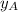 is the mole fraction in the vapor
is the mole fraction in the vapor