Chemistry, 06.05.2020 03:28 alwaysneedhelp84
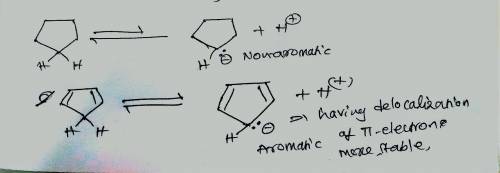
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded to an sp3sp3 carbon. Explain why cyclopentadiene is a much stronger acid (pKapKa of 15), even though it too involves the loss of a proton from an sp3sp3 carbon. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded...
Questions

Health, 21.03.2020 00:35

Mathematics, 21.03.2020 00:35

Computers and Technology, 21.03.2020 00:35



Physics, 21.03.2020 00:35

Mathematics, 21.03.2020 00:35


Mathematics, 21.03.2020 00:35

Mathematics, 21.03.2020 00:35


Biology, 21.03.2020 00:35

Social Studies, 21.03.2020 00:35

Mathematics, 21.03.2020 00:35

Biology, 21.03.2020 00:35



Mathematics, 21.03.2020 00:35

Mathematics, 21.03.2020 00:35