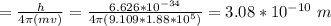Chemistry, 06.05.2020 01:59 silvajimena74
An electron travelling at 3.7 x 105 m/s has an uncertainty in its velocity of 1.88 x 105 m/s. What is the uncertainty in its position (in pm)? (Just put the number, don't use scientific notation and decimal point in the final answer) Avogadro's number = 6.022 x 1023, h = 6.626 x 10-34 J. s, c = 3.00 x 108 m/s, me= 9.109 x 10-31 kg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1


You know the right answer?
An electron travelling at 3.7 x 105 m/s has an uncertainty in its velocity of 1.88 x 105 m/s. What i...
Questions




English, 14.02.2020 04:15