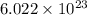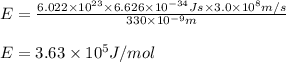Chemistry, 06.05.2020 02:01 mildred3645
An emission line of sodium has a wavelength of 330 nm. Calculate the energy of a photon of light emitted in J/ atom, and the energy emitted per mole of Na atoms at this wavelength.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 20:40
Who discovered that a dense positively charged atomic nucleus is at the core of every atom
Answers: 1
You know the right answer?
An emission line of sodium has a wavelength of 330 nm. Calculate the energy of a photon of light emi...
Questions


History, 30.04.2021 23:30



Mathematics, 30.04.2021 23:30

History, 30.04.2021 23:30

Computers and Technology, 30.04.2021 23:30

Mathematics, 30.04.2021 23:30


Mathematics, 30.04.2021 23:30



Mathematics, 30.04.2021 23:30


Biology, 30.04.2021 23:30


Biology, 30.04.2021 23:30



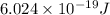 and energy emitted per mole of sodium atoms is
and energy emitted per mole of sodium atoms is 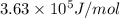
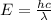
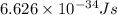
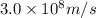
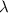 = wavelength of photon = 330 nm =
= wavelength of photon = 330 nm = 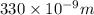 (Conversion factor:
(Conversion factor: 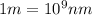 )
)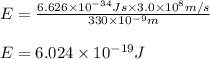
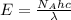
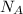 = Avogadro's number =
= Avogadro's number =