
Chemistry, 06.05.2020 02:14 vandarughb5653
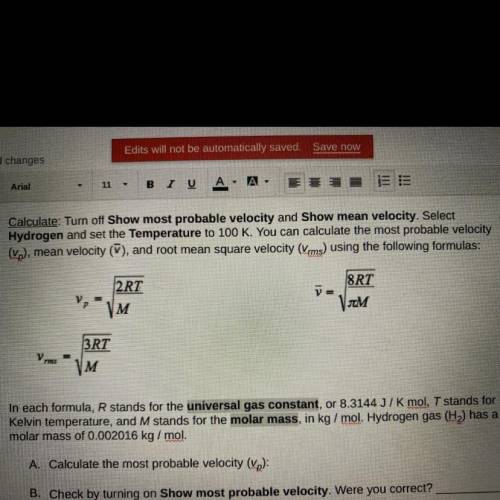
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelvin temperature, and M stands for the molar mass, in kg/mol. Hydrogen gas (H) has a
molar mass of 0.002016 kg/mol.
A. Calculate the most probable velocity (vp):
B. Check by turning on Show most probable velocity. Were you correct?
C. Calculate the mean velocity (V):
D. Check by turning on Show mean velocity. Were you correct?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelv...
Kelv...
Questions

Mathematics, 12.07.2021 20:30


Mathematics, 12.07.2021 20:30

English, 12.07.2021 20:40

Social Studies, 12.07.2021 20:40



Mathematics, 12.07.2021 20:40


English, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40

Social Studies, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40



Mathematics, 12.07.2021 20:40


Mathematics, 12.07.2021 20:40



