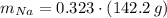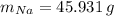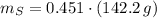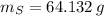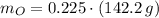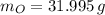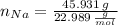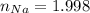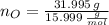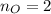Chemistry, 06.05.2020 02:23 BrodsterBj
Calcula la fórmula molecular de un compuesto formado por 32.3% de sodio, 45.1% de azufre y 22.5% de oxígeno, el peso molecular del compuesto es de 142.2 gr

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Calcula la fórmula molecular de un compuesto formado por 32.3% de sodio, 45.1% de azufre y 22.5% de...
Questions

Computers and Technology, 18.09.2019 14:00




Mathematics, 18.09.2019 14:00

Social Studies, 18.09.2019 14:00



Chemistry, 18.09.2019 14:00


Mathematics, 18.09.2019 14:00







Chemistry, 18.09.2019 14:00

History, 18.09.2019 14:00

Social Studies, 18.09.2019 14:00

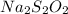 .
.