Chemistry, 06.05.2020 00:15 dpinzoner5952
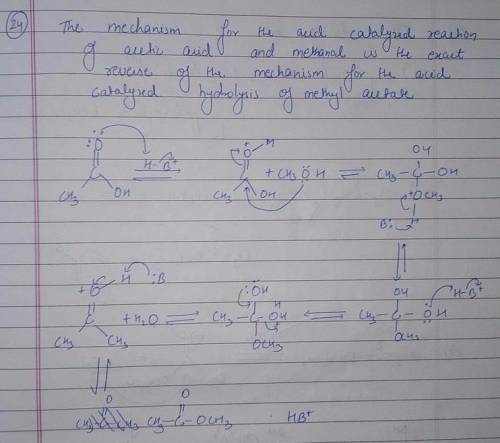
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism-showing all the curved arrows-for the acid-catalyzed reaction of acetic acid and methanol to form methyl acetate. All proton-donating and proton-removing species are given on canvas.
there are 7 boxesO
||
the first one has H3C-C-OH with OH+ --H
O
||
and the final product is H3C-C-O-CH3 with H3O+

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism...
Questions



Mathematics, 27.06.2020 15:01



Engineering, 27.06.2020 15:01


Social Studies, 27.06.2020 15:01

Social Studies, 27.06.2020 15:01

Physics, 27.06.2020 15:01


Mathematics, 27.06.2020 15:01

Mathematics, 27.06.2020 15:01




Chemistry, 27.06.2020 15:01



Mathematics, 27.06.2020 15:01