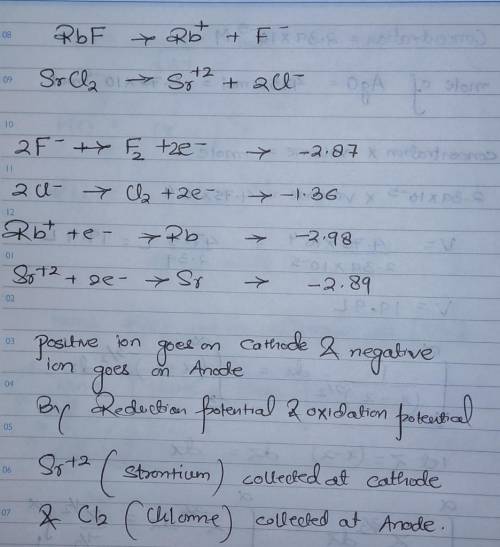Chemistry, 06.05.2020 00:22 osmanysalvador9
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the negative electrode and at the positive electrode. The cell temperature must be high enough to keep the salt mixture molten hence the metal appears as a liquid and the halogen as a gas. product at the negative electrode (cathode) product at the positive electrode (anode)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the neg...
Questions

Mathematics, 06.11.2020 22:00

Spanish, 06.11.2020 22:00



Mathematics, 06.11.2020 22:00

History, 06.11.2020 22:00


Mathematics, 06.11.2020 22:00

Biology, 06.11.2020 22:00





Geography, 06.11.2020 22:00

Mathematics, 06.11.2020 22:00


English, 06.11.2020 22:00


SAT, 06.11.2020 22:00