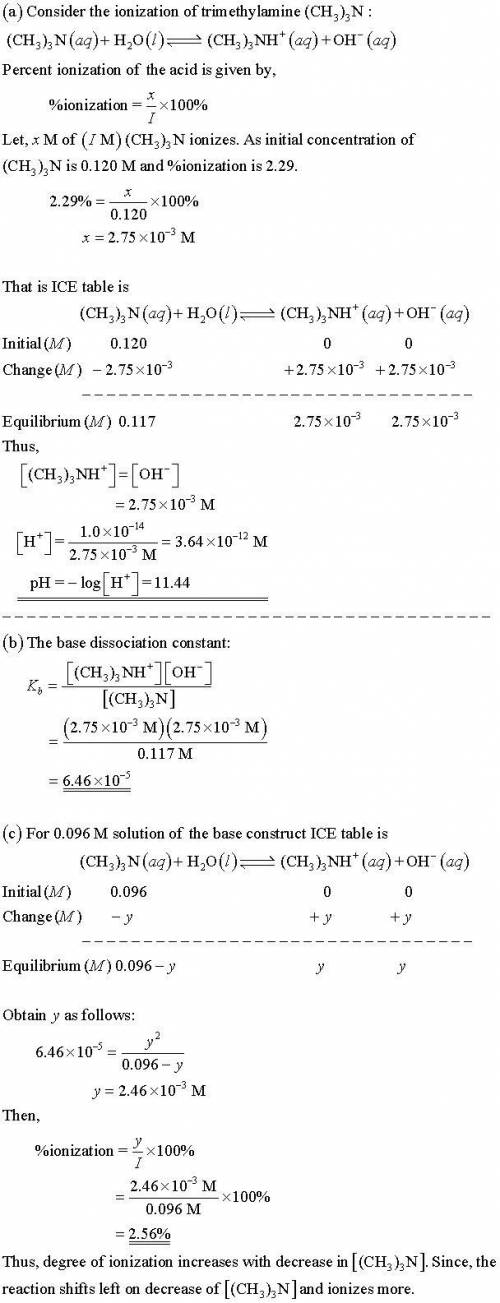
Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution:
(CH3)3N(aq) + H2O(l)...

Chemistry, 06.05.2020 00:18 aliciapinto13
Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution:
(CH3)3N(aq) + H2O(l) (CH3)3NH+(aq) + OH−(aq)
A 0.120 M solution of (CH3)3N(aq) is 2.29% ionized at 25OC.
(a) Calculate [OH− ], [(CH3)3NH+ ], [H3O+ ] and the pH for a 0.120 M (CH3)3N(aq)
solution at 25oC.
(b) Calculate Kb for (CH3)3N at 25oC.
(c) Calculate the degree of ionization, α, of a 0.096 M solution of trimethylamine. Does
the degree of ionization increase, decrease, or remain unchanged as the concentration
of (CH3)3N decreases? Give reasons for your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
Questions

English, 28.10.2020 02:40

Mathematics, 28.10.2020 02:40

Mathematics, 28.10.2020 02:40

Physics, 28.10.2020 02:40


History, 28.10.2020 02:40



Mathematics, 28.10.2020 02:40



Mathematics, 28.10.2020 02:40


Mathematics, 28.10.2020 02:40

Spanish, 28.10.2020 02:40

Mathematics, 28.10.2020 02:40


Biology, 28.10.2020 02:40

Mathematics, 28.10.2020 02:40

Computers and Technology, 28.10.2020 02:40