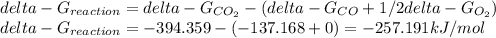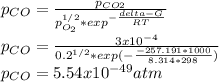Chemistry, 06.05.2020 00:21 Jenniferojeda2002
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The partial pressure of O2(g) is 0.2 bar and the partial pressure of CO2(g) is 3 * 10-4 bar. CO is extremely poisonous because it forms a very strong complex with hemoglobin. Should you worry?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The...
Questions





Mathematics, 07.01.2021 07:20

Mathematics, 07.01.2021 07:20

Mathematics, 07.01.2021 07:20


Mathematics, 07.01.2021 07:20

Mathematics, 07.01.2021 07:20

Mathematics, 07.01.2021 07:20

English, 07.01.2021 07:20



English, 07.01.2021 07:20




Mathematics, 07.01.2021 07:30

Mathematics, 07.01.2021 07:30