
Chemistry, 05.05.2020 22:04 williamrobinson93
Use standard entropies to calculate δs∘rxn for the balanced chemical equation: 2pcl3(g)+o2(g)→2pocl3(l) substance s∘(j/mol⋅k) pocl3(l) 222.5 pocl3(g) 325.5 pcl3(l) 217.1 pcl3(g) 311.8 o2(g) 205.2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Use standard entropies to calculate δs∘rxn for the balanced chemical equation: 2pcl3(g)+o2(g)→2pocl3...
Questions






Computers and Technology, 07.06.2021 05:50


Geography, 07.06.2021 05:50



Mathematics, 07.06.2021 05:50


Mathematics, 07.06.2021 05:50

Computers and Technology, 07.06.2021 05:50

Mathematics, 07.06.2021 06:00





Mathematics, 07.06.2021 06:00

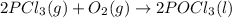
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(CO_2(g))})]-[(1\times \Delta S^o_{(O_2(g))})+(2\times \Delta S_{(PCl_3(g))})]](/tpl/images/0643/0247/f3c7b.png)
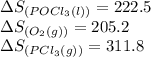
![\Delta S^o_{rxn}=[(2\times (222.5))]-[(1\times 205.2)+(2\times (311.8))]](/tpl/images/0643/0247/90f90.png)


