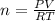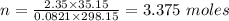Chemistry, 05.05.2020 21:21 janiyahmcgolley
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reaction:
CH4 (s) + 2 O2 (g) --> CO2 (g) + 2 H2O (l)
What mass of methane (in grams) will require 35.15 L of oxygen to fully react? The pressure is 2.35 atm and the temperature is 15 °C. (R = 0.0821 L atm/mol K)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reactio...
Questions




Mathematics, 26.08.2019 08:50

Chemistry, 26.08.2019 08:50

Mathematics, 26.08.2019 08:50

English, 26.08.2019 08:50


Social Studies, 26.08.2019 08:50

Social Studies, 26.08.2019 08:50



History, 26.08.2019 08:50


Mathematics, 26.08.2019 08:50

Biology, 26.08.2019 08:50

Computers and Technology, 26.08.2019 08:50



Biology, 26.08.2019 08:50