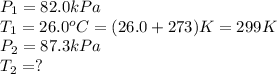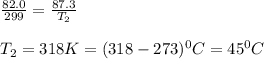Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
A car tire was inflated to 82.0 kPa in a repair shop where the temperature is 26.0 °c. What is the t...
Questions

English, 19.10.2019 14:50

Physics, 19.10.2019 14:50





Mathematics, 19.10.2019 14:50

Chemistry, 19.10.2019 14:50

Mathematics, 19.10.2019 14:50


Mathematics, 19.10.2019 14:50


Social Studies, 19.10.2019 14:50




History, 19.10.2019 14:50


Mathematics, 19.10.2019 14:50

Mathematics, 19.10.2019 14:50

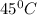
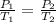
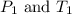 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.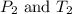 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.