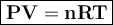Chemistry, 13.01.2020 01:31 natetheman7740
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl(aq), as described by the chemical equation.
how much mno2(s) should be added to excess hcl(aq) to obtain 185 ml of cl2(g) at 25 °c and 715 torr?
chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl(aq), as described by the chemical equation.
how much mno2(s) should be added to excess hcl(aq) to obtain 185 ml of cl2(g) at 25 °c and 715 torr?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1



Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

Mathematics, 08.04.2020 06:19

Mathematics, 08.04.2020 06:19

Health, 08.04.2020 06:19


Biology, 08.04.2020 06:20

Social Studies, 08.04.2020 06:20




Mathematics, 08.04.2020 06:20

Mathematics, 08.04.2020 06:20



Mathematics, 08.04.2020 06:20

Mathematics, 08.04.2020 06:21

English, 08.04.2020 06:22

Mathematics, 08.04.2020 06:22



Health, 08.04.2020 06:23