
Chemistry, 05.05.2020 19:24 elpancho755
Balance the equation by inserting coefficients as needed. equation: ZnS + HBr -> ZnBr_{2} + H_{2}S ZnS + HBr ⟶ ZnBr 2 + H 2 S

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Balance the equation by inserting coefficients as needed. equation: ZnS + HBr -> ZnBr_{2} + H_{2}...
Questions


History, 14.11.2019 23:31







Health, 14.11.2019 23:31

Chemistry, 14.11.2019 23:31

Mathematics, 14.11.2019 23:31

Business, 14.11.2019 23:31


History, 14.11.2019 23:31




History, 14.11.2019 23:31

Business, 14.11.2019 23:31

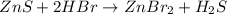
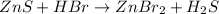
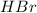 and we get the balanced chemical equation.
and we get the balanced chemical equation.

