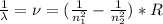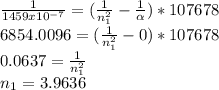Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagnetic spectrum. Each series fits the Rydberg equation with its own particular n1 value. Calculate the value of n1 that would produce a series of lines in which the highest energy line has a wavelength of 1459 nm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagn...
Questions


Mathematics, 31.10.2021 06:00

Mathematics, 31.10.2021 06:00

Chemistry, 31.10.2021 06:00

Mathematics, 31.10.2021 06:00

Mathematics, 31.10.2021 06:00

Mathematics, 31.10.2021 06:00

Mathematics, 31.10.2021 06:00

Health, 31.10.2021 06:00




Biology, 31.10.2021 06:10


Mathematics, 31.10.2021 06:10

Chemistry, 31.10.2021 06:10


Mathematics, 31.10.2021 06:10