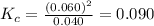Chemistry, 05.05.2020 18:04 AgentPangolin
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2


You know the right answer?
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350...
Questions

Computers and Technology, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10


Mathematics, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10




Chemistry, 09.12.2020 01:10

Health, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10

English, 09.12.2020 01:10

History, 09.12.2020 01:10


Mathematics, 09.12.2020 01:10

Spanish, 09.12.2020 01:10

Mathematics, 09.12.2020 01:10

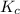 is 0.090.
is 0.090.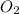 =
= 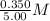 = 0.0700 M
= 0.0700 M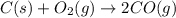
![K_{c}=\frac{[CO]^{2}}{[O_{2}]}](/tpl/images/0641/0273/4df57.png) , where [CO] and
, where [CO] and ![[O_{2}]](/tpl/images/0641/0273/9a638.png) represents equilibrium concentration of CO and
represents equilibrium concentration of CO and ![[CO]=2x=0.060](/tpl/images/0641/0273/522d6.png)