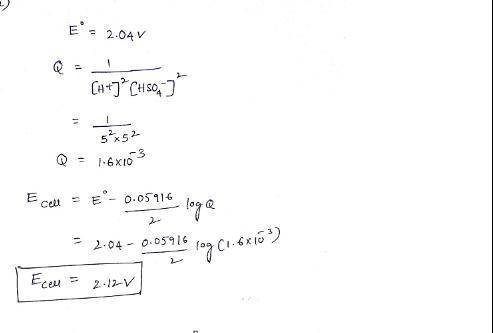Chemistry, 05.05.2020 18:14 kenleighbrooke67
The overall reaction in the lead storage battery is
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(aq) > 2PbSO4(s) + 2H2O(l)
Calculate % at 258C for this battery when [H2SO4] 5 4.5 M; that is, [H1] 5 [HSO42] 5 4.5 M. At 258C, %8 5 2.04 V for the lead storage battery.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
The overall reaction in the lead storage battery is
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(...
Pb(s) + PbO2(s) + 2H1(aq) + 2HSO42(...
Questions


Biology, 06.06.2021 14:00

Arts, 06.06.2021 14:00






Chemistry, 06.06.2021 14:00

Biology, 06.06.2021 14:00

English, 06.06.2021 14:00


English, 06.06.2021 14:00

Computers and Technology, 06.06.2021 14:00


Social Studies, 06.06.2021 14:00

Physics, 06.06.2021 14:00

Biology, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00

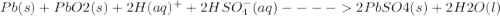
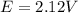
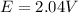
![Q = \frac{1}{[[HSO_4^-]^2 [H^+] ^2]}](/tpl/images/0641/1298/ac965.png)
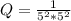

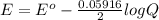
![= 2.04 - [\frac{0.05916}{2} log (1.6*10^{-3})]](/tpl/images/0641/1298/8888a.png)