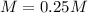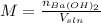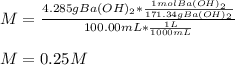Chemistry, 05.05.2020 17:05 jholland18
A solution of barium hydroxide Ba(OH) contains 4.285 g of barium hydroxide in 100.0 mL of solution. What is molarity of the solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 13:30
The dashed segment of the plotted experiment in the graph in the lesson is called an: interpolation extrapolation extension assumption
Answers: 1
You know the right answer?
A solution of barium hydroxide Ba(OH) contains 4.285 g of barium hydroxide in 100.0 mL of solution....
Questions


Social Studies, 01.12.2021 19:40



Chemistry, 01.12.2021 19:40



Mathematics, 01.12.2021 19:40



Mathematics, 01.12.2021 19:40



Biology, 01.12.2021 19:40

Mathematics, 01.12.2021 19:40

SAT, 01.12.2021 19:40

Mathematics, 01.12.2021 19:40


Business, 01.12.2021 19:40

Computers and Technology, 01.12.2021 19:40