Chemistry, 05.05.2020 17:25 morganhines181
Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.80 g Al is mixed with 0.23 g Cl2. (a) What is the limiting reactant? Cl2 Al (b) What is the maximum amount of AlCl3, in grams, that can be produced? g AlCl3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine g...
Questions




Mathematics, 12.02.2020 02:03

Mathematics, 12.02.2020 02:03



Mathematics, 12.02.2020 02:03

Mathematics, 12.02.2020 02:03




Mathematics, 12.02.2020 02:03


Mathematics, 12.02.2020 02:03





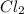 is the limiting reagent
is the limiting reagent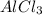 will be produced.
will be produced.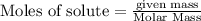
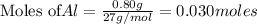
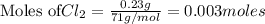
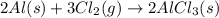
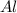
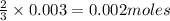 of
of