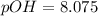Chemistry, 05.05.2020 16:09 alexabrandon1848
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M solution of HCl at 25°C. Enter your numbers to 2 decimal places. Kb = 4.4x10-4 What is the pH of the methylamine solution before titrant is added? 11.91 How many milliliters of titrant are required to reach the equivalence point? 36.59 What is the pH at the equivalence point?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M soluti...
Questions

Mathematics, 24.08.2021 03:30


Mathematics, 24.08.2021 03:30

Health, 24.08.2021 03:30




Computers and Technology, 24.08.2021 03:30



Social Studies, 24.08.2021 03:30








English, 24.08.2021 03:30

![pOH = \frac{1}{2}[pK_b \ - \ log \ C]](/tpl/images/0640/0162/873d4.png)
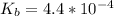
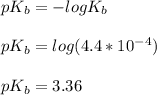
![pOH = \frac{1}{2}[3.36\ - \ log \0.15]](/tpl/images/0640/0162/71cf9.png)
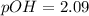
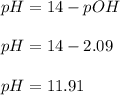
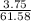
![pOH = \frac{1}{2}[pK_w+pK_b+log \ C]](/tpl/images/0640/0162/af00e.png)
![pOH = \frac{1}{2}[14+3.36+log \ 0.061]](/tpl/images/0640/0162/443df.png)
![pOH = \frac{1}{2}[16.15]](/tpl/images/0640/0162/bf604.png)