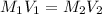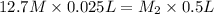Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1


Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
You know the right answer?
A stock solution of HNO3HNO3 is prepared and found to contain 12.7 M of HNO3HNO3. If 25.0 mL of the...
Questions

Mathematics, 27.04.2021 23:10

Mathematics, 27.04.2021 23:10






Mathematics, 27.04.2021 23:10


Mathematics, 27.04.2021 23:10

Mathematics, 27.04.2021 23:10




Mathematics, 27.04.2021 23:10



History, 27.04.2021 23:10

Mathematics, 27.04.2021 23:10

History, 27.04.2021 23:10

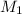 = 12.7 M,
= 12.7 M, 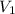 = 25.0 ml = 0.025 L (as 1 ml = 0.001 L)
= 25.0 ml = 0.025 L (as 1 ml = 0.001 L)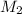 = ? ,
= ? , 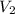 = 0.5 L
= 0.5 L