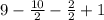Chemistry, 05.05.2020 16:28 giiiselleee05
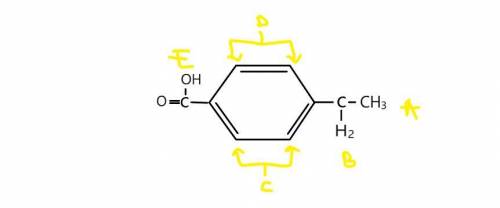
Question 23Get help answering Molecular Drawing questions Get help answering Molecular Drawing questions. A compound with molecular formula C9H10O2 exhibits a triplet at δ 1.2 (3H), a quartet at δ 2.6 (2H), a doublet at δ 7.3 (2H), a doublet at δ 8.0 (2H) and a singlet at δ 11 (1H) in its 1HNMR spectrum. What is the structure for this compound?MarvinJS Response ImageEdit

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Question 23Get help answering Molecular Drawing questions Get help answering Molecular Drawing quest...
Questions

Biology, 19.01.2021 06:10



Mathematics, 19.01.2021 06:10


French, 19.01.2021 06:10


English, 19.01.2021 06:10






History, 19.01.2021 06:10


Social Studies, 19.01.2021 06:10




History, 19.01.2021 06:10

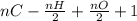 (where n = no of moles)
(where n = no of moles)