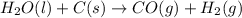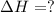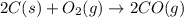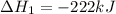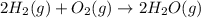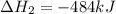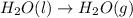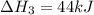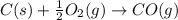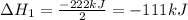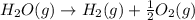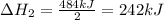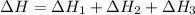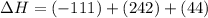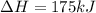Chemistry, 05.05.2020 16:29 shawn20034
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemical data above to calculate the change in enthalpy for the reaction below. H2O(l)+C(s)→CO(g)+H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemic...
Questions




Mathematics, 12.02.2021 08:20


Mathematics, 12.02.2021 08:20

Mathematics, 12.02.2021 08:20


Mathematics, 12.02.2021 08:20

Spanish, 12.02.2021 08:20


Mathematics, 12.02.2021 08:20





Mathematics, 12.02.2021 08:20

History, 12.02.2021 08:20

Mathematics, 12.02.2021 08:20

Biology, 12.02.2021 08:20