
Chemistry, 05.05.2020 16:30 hhhhhh8897
21-B. Mn was used as an internal standard for measuring Fe by atomic absorption. A standard mixture containing 2.00 mg Mn/mL and 2.50 mg Fe/mL gave a quotient (Fe signal/Mn signal) 5 1.05/1.00. A mixture with a volume of 6.00 mL was prepared by mixing 5.00 mL of unknown Fe solution with 1.00 mL containing 13.5 mg Mn/mL. The absorbance of this mixture at the Mn wave- length was 0.128, and the absorbance at the Fe wavelength was 0.185. Find the molarity of the unknown Fe solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
21-B. Mn was used as an internal standard for measuring Fe by atomic absorption. A standard mixture...
Questions

Mathematics, 12.06.2021 03:00

Mathematics, 12.06.2021 03:00


Mathematics, 12.06.2021 03:00

Physics, 12.06.2021 03:00


Mathematics, 12.06.2021 03:00






Social Studies, 12.06.2021 03:00

Mathematics, 12.06.2021 03:00



English, 12.06.2021 03:00

English, 12.06.2021 03:00


Mathematics, 12.06.2021 03:00

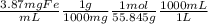 = 0.0693M Fe
= 0.0693M Fe

