Chemistry, 05.05.2020 16:32 homeworkprincess
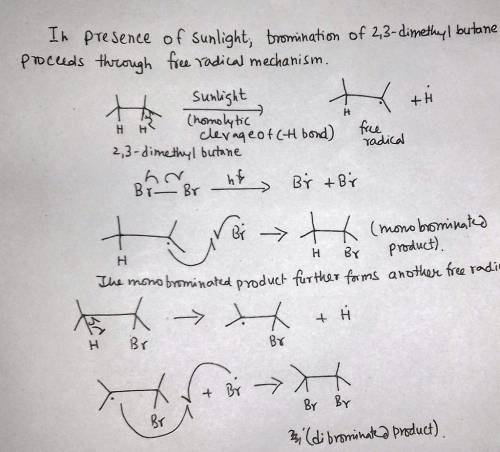
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these products, and propose a mechanism for the formation of the monobrominated product.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1
You know the right answer?
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Fu...
Questions

Mathematics, 16.09.2019 05:10

Social Studies, 16.09.2019 05:10





Mathematics, 16.09.2019 05:10

English, 16.09.2019 05:20


Spanish, 16.09.2019 05:20

History, 16.09.2019 05:20

Mathematics, 16.09.2019 05:20


Mathematics, 16.09.2019 05:20

Chemistry, 16.09.2019 05:20

Mathematics, 16.09.2019 05:20


History, 16.09.2019 05:20

Mathematics, 16.09.2019 05:20

Chemistry, 16.09.2019 05:20