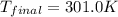Chemistry, 05.05.2020 15:04 BeautyxQueen
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an insulated container.
What is the final temperature of both substances at thermal equilibrium?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an...
Questions

Mathematics, 28.09.2020 14:01

Biology, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01



Business, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Social Studies, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01


Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


English, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

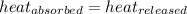
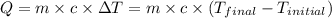
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0639/3665/09236.png) .................(1)
.................(1)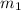 = mass of gold = 31.5 g
= mass of gold = 31.5 g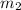 = mass of water = 63.4 g
= mass of water = 63.4 g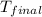 = final temperature = ?
= final temperature = ?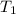 = temperature of gold =
= temperature of gold = 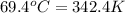
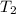 = temperature of water =
= temperature of water = 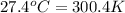
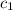 = specific heat of gold =
= specific heat of gold = 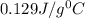
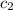 = specific heat of water=
= specific heat of water= 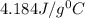
![-31.5\times 0.129\times (T_{final}-342.4)=[63.4\times 4.184\times (T_{final}-300.4)]](/tpl/images/0639/3665/eb953.png)