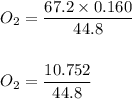Chemistry, 05.05.2020 15:06 rmans22209
Assuming that pressure and temperature are constant, how many liters of oxygen gas will be required to produce 0.160 L of nitrogen gas.
4 NH3 (g) + 3 O2 (g) → 6 H2O (g) + 2 N2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
Assuming that pressure and temperature are constant, how many liters of oxygen gas will be required...
Questions

Mathematics, 30.04.2021 17:40

Mathematics, 30.04.2021 17:40

History, 30.04.2021 17:40

Mathematics, 30.04.2021 17:40


History, 30.04.2021 17:40


Chemistry, 30.04.2021 17:40



Spanish, 30.04.2021 17:40



History, 30.04.2021 17:40




Health, 30.04.2021 17:40

English, 30.04.2021 17:40

Social Studies, 30.04.2021 17:40