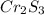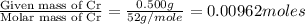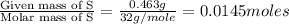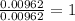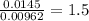Chemistry, 05.05.2020 15:08 teionamwhite2262
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate the empirical formula of the chromium sulfide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate...
Questions

Mathematics, 16.10.2020 17:01


History, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01


English, 16.10.2020 17:01





Physics, 16.10.2020 17:01


Computers and Technology, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01


Biology, 16.10.2020 17:01


English, 16.10.2020 17:01