
Chemistry, 05.05.2020 15:17 klivingston1012
How many moles of sulfuric acid are required to make (4.0x10^2) mL of (1.140x10^-1) mol/L solution?
* Express your answer in scientific notation to the correct number of significant digits. Remember th:
scientific notation has one non-zero number to the left of the decimal point (i. e.: 1.23 x 104).
Note: Your answer is assumed to be reduced to the highest power possible.
Your
X10
Answer
units

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
How many moles of sulfuric acid are required to make (4.0x10^2) mL of (1.140x10^-1) mol/L solution?<...
Questions

History, 01.03.2020 04:05

History, 01.03.2020 04:05







Physics, 01.03.2020 04:06



Mathematics, 01.03.2020 04:06

Mathematics, 01.03.2020 04:06



Mathematics, 01.03.2020 04:06

Mathematics, 01.03.2020 04:07

Mathematics, 01.03.2020 04:07


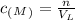 where n is the moles of the solute, and V is the volume of the solution in liters (L).
where n is the moles of the solute, and V is the volume of the solution in liters (L).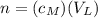
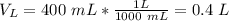
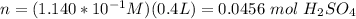
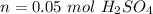 (rounded)
(rounded)


