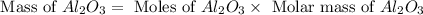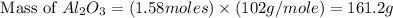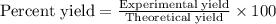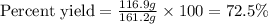This question involves two calculations. The answer to the first part will be
used for calculating the second part.
Aluminum powder, Al, will react and burn with oxygen gas, O2, to produce
aluminum oxide, Al2O3, according to the following balanced equation:
4 Al + 302
+ 2Al2O3
a) If 85.1 grams of aluminum react with excess oxygen in the air, how many
grams of aluminum oxide, Al2O3, will theoretically be produced?
b) If after the reaction is completed, 116.9 g of Al2O3 were actually
recovered and measured, what is the percent yield of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
This question involves two calculations. The answer to the first part will be
used for calcula...
used for calcula...
Questions





Mathematics, 29.08.2020 01:01






History, 29.08.2020 01:01

Chemistry, 29.08.2020 01:01



Health, 29.08.2020 01:01

Biology, 29.08.2020 01:01

English, 29.08.2020 01:01


Social Studies, 29.08.2020 01:01

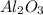 produced is, 15.2 grams.
produced is, 15.2 grams.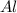 = 85.1 g
= 85.1 g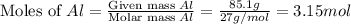
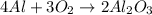
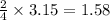 mole of
mole of