
Chemistry, 05.05.2020 10:37 googoomylizard
There are 231.0 grams of Iron (ll) Hydroxide, Fe(OH)2. How many moles of Fe(OH)2 does that equal?
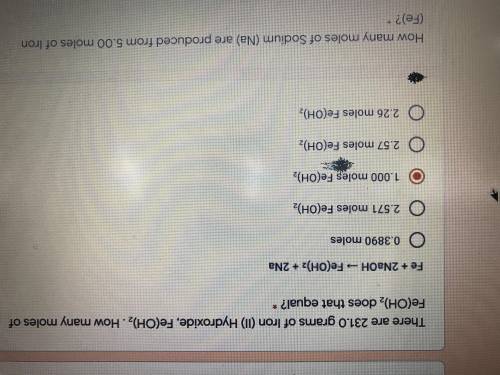

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

You know the right answer?
There are 231.0 grams of Iron (ll) Hydroxide, Fe(OH)2. How many moles of Fe(OH)2 does that equal?
Questions

History, 11.11.2020 01:00



Geography, 11.11.2020 01:00

Social Studies, 11.11.2020 01:00




Mathematics, 11.11.2020 01:00

History, 11.11.2020 01:00




History, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Arts, 11.11.2020 01:00

English, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00



