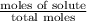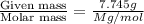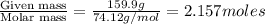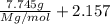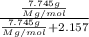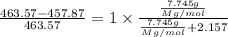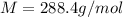The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. The vapor pressure of diethyl ether , CH3CH2OCH2CH3, is 463.57 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 7.745 grams of the compound were dissolved in 159.9 grams of diethyl ether, the vapor pressure of the solution was 457.87 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in...
Questions


Mathematics, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10



Mathematics, 14.07.2019 11:10

History, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

History, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

Health, 14.07.2019 11:10


Mathematics, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

History, 14.07.2019 11:10

English, 14.07.2019 11:10

Mathematics, 14.07.2019 11:10

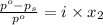
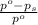 = relative lowering in vapor pressure
= relative lowering in vapor pressure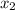 = mole fraction of solute =
= mole fraction of solute =