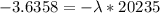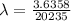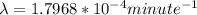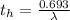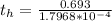Be sure to answer all parts. A freshly isolated sample of 90Y was found to have an activity of 2.2 × 105 disintegrations per minute at 1:00 p. m. on December 3, 2006. At 2:15 p. m. on December 17, 2006, its activity was measured again and found to be 5.8 × 103 disintegrations per minute. Calculate the half-life of 90Y. Enter your answer in scientific notation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Be sure to answer all parts. A freshly isolated sample of 90Y was found to have an activity of 2.2 ×...
Questions


Mathematics, 27.01.2021 02:50




Chemistry, 27.01.2021 02:50



English, 27.01.2021 02:50

Social Studies, 27.01.2021 02:50

Social Studies, 27.01.2021 02:50



Mathematics, 27.01.2021 02:50



History, 27.01.2021 02:50



Geography, 27.01.2021 02:50


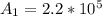 per minute
per minute 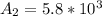 per minute
per minute 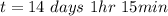
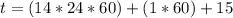
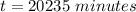
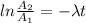
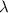 is the rate constant
is the rate constant ![ln [\frac{5.8 * 10^{3}}{2.2 *10^{5}} ] = - \lambda * 20235](/tpl/images/0634/6329/6ece6.png)