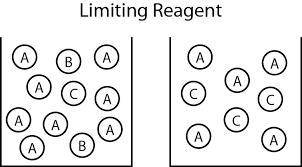
Chemistry, 05.05.2020 07:10 ghanim1963
An aqueous solution containing 9.82 g9.82 g of lead(II) nitrate is added to an aqueous solution containing 5.76 g5.76 g of potassium chloride. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. balanced chemical equation: Pb(NO3)2(aq)+2KCl(aq)⟶PbCl2(s)+2KNO 3(aq)Pb(NO3)2(aq)+2KCl(aq)⟶PbCl2(s) +2KNO3(aq) What is the limiting reactant? potassium chloride lead(II) nitrate The percent yield for the reaction is 87.5%87.5% . How many grams of the precipitate are formed? precipitate formed: gg How many grams of the excess reactant remain?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
An aqueous solution containing 9.82 g9.82 g of lead(II) nitrate is added to an aqueous solution cont...
Questions



Social Studies, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01


Mathematics, 15.10.2020 01:01



Social Studies, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01



History, 15.10.2020 01:01



Chemistry, 15.10.2020 01:01



English, 15.10.2020 01:01

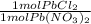 *
* 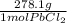 * 87.5/100 = 7.20 g PbCl₂
* 87.5/100 = 7.20 g PbCl₂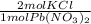 *
* 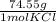 = 3.86 g KCl
= 3.86 g KCl