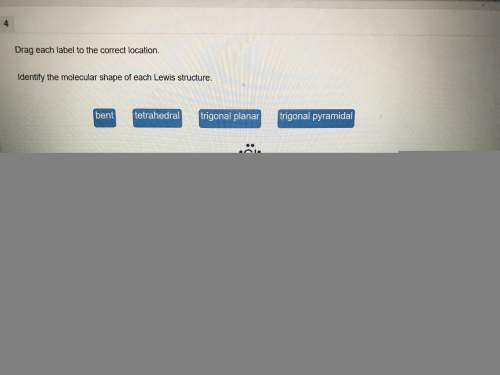
PLZ HELP ASAP
A student must use 225 g of hot water in a lab procedure. Calculate the amount...

Chemistry, 05.05.2020 07:30 HeroesofOlympus96951
PLZ HELP ASAP
A student must use 225 g of hot water in a lab procedure. Calculate the amount of heat in joules required to raise the temperature of 225 g of water from 20.0 °C to 100.0 °C. (Water’s specific heat capacity is 4.184 J/g°C) (round answer to 2-3 sig figs)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3
You know the right answer?
Questions




English, 11.10.2019 16:10














Computers and Technology, 11.10.2019 16:10