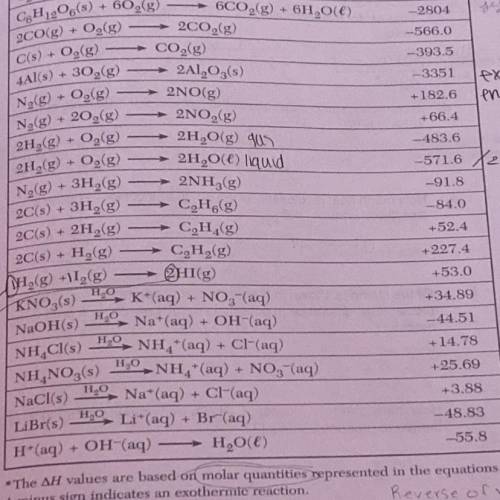
How much heat is released when 80 grams of NaOh (molar mass= 40 g) dissolve in water?
H...

How much heat is released when 80 grams of NaOh (molar mass= 40 g) dissolve in water?
How many grams of glucose C6H12O6 (molar mass= 180g) must be burned to produce 180.4 KJ?
How much heat is absorbed in the formation of 1.00 mole of HI (g) from H2 (g) and I2 (g)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
Questions


Mathematics, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06



Chemistry, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06



History, 06.05.2020 08:06

Chemistry, 06.05.2020 08:06


Mathematics, 06.05.2020 08:06

History, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06


Mathematics, 06.05.2020 08:06

Mathematics, 06.05.2020 08:06

Biology, 06.05.2020 08:06



